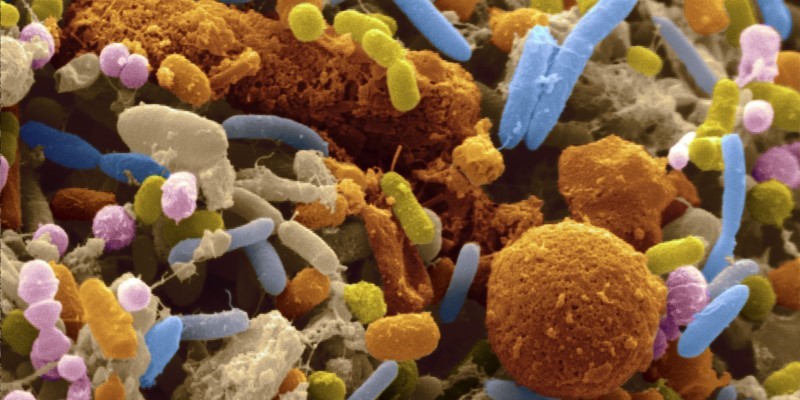
The human body is inhabited by many and varied microbial species. These include bacteria, viruses and fungi, and collectively they are referred to as the ‘microbiota’. Generally, the microbiota is thought to be highly beneficial to human (and animal) health, aiding immunity, digestion and even mental health. In the face of infectious disease, the microbiota is known to play an important role in fending off disease-causing microbes. However, in certain conditions, the microbiota can also facilitate infection. For example, when a person’s immunity becomes compromised through illness, injury or surgery, microbiota components can exploit this reduced immunity and become the cause of disease. In such cases, these microbes are referred to as ‘opportunistic pathogens’. A well-known opportunist is the bacterium Staphylococcus aureus, better known in its antibiotic-resistant form as the hospital superbug MRSA (methicillin-resistant S. aureus). Around 30% of the human population carries S. aureus in the nose, where it can live happily as a component of the microbiota without causing disease. Only when its host falls into a state of depleted health, becoming vulnerable to infection, does this bacterium transition from harmless microbiota component to opportunistic pathogen. Understanding the triggers of these microbial transitions is a key aspect in understanding how infections become established in a susceptible patient. Furthermore, by improving our understanding of the drivers of bacterial pathogenesis, we can work towards improved prevention and treatment of infectious disease.
In the face of rapidly rising antibiotic resistance levels around the world, this is becoming increasingly important. S. aureus is listed among a group of 6 bacterial pathogens (the ESKAPE pathogens) currently exhibiting multi-drug resistance in hospital settings. As antibiotics become less effective in treating bacterial disease, there is a pressing need to develop alternative treatments. One approach which holds a lot of promise is the potential to use beneficial microbiota components at the site of infection, to outcompete or fight off pathogens and ultimately cure infection. While this might sound straightforward, there are many things to consider before such an approach becomes widely implemented. Primarily, there are safety concerns about transplanting living microbes between patients; something that is harmless in one person could become the cause of opportunistic infection in another. A further concern is how our microbial inhabitants – both harmless microbiota and pathogens – might respond in evolutionary terms. Could future introduction of beneficial microbes to a new patient drive evolutionary change in pathogens towards greater virulence? Could beneficial microbes used for disease treatment transition to become opportunists?
These are the questions I am trying to answer. I am investigating how the opportunist S. aureus responds over evolutionary time to the presence or absence of a host microbiota. Interestingly, in a nematode worm host, I am finding that presence of an otherwise harmless microbiota community makes S. aureus infection worse. I am currently investigating the evolutionary trajectory of S. aureus in response to host microbiota, to determine how otherwise beneficial microbes might (positively or negatively) contribute to the evolution of pathogen virulence. In doing so, I aim to help move forward current understanding of the evolutionary drivers of pathogen virulence, and investigate the potential implications for using beneficial microbes as a form of treatment for infectious disease.
Emily Stevens is currently a post-doctoral researcher in the Department of Zoology (soon to be the Department of Biology) and holds an EPA Cephalosporin JRF at Linacre College. She completed my PhD in microbiology at the University of Bristol in 2019.